What is Cleaning in Place (CIP)?
CIP means Clean in Place. It describes automatic cleaning without the disassembly of production equipment on site, for example in the beverage and food industry. CIP systems can be used to remove production-related deposits and contamination from tanks as well as piping systems, among other areas in which contaminants may occur. In this process, the areas and objects to be cleaned are connected in a circuit with a CIP system. The cleaning sequence is individually adapted to meet the cleaning requirements of the object in question and to the degree of contamination.
Why is CIP cleaning important?
Production -surfaces in contact with the product may retain products-residues which can serve as growth medium for microorganisms. If harmful microorganisms are not regularly removed by cleaning and disinfection processes in your plant, product safety and/or product quality will suffer. Depending on the contamination, you may be faced with shortened minimum shelf lives or even product recalls.
To prevent this, efficient cleaning and decontamination of all plant components is of utmost importance. However, complex geometries such as piping, valves, internals in tanks or heat exchangers in closed systems are not accessible at all or only with difficulty. Automated CIP allows you to save time and money by eliminating costly and manual cleaning procedures while improving hygiene in your systems. This applies equally to open systems such as storage tanks. Successful cleaning is an essential aspect in guaranteeing a high level of microbiological safety for your products and thus contributes to quality assurance.
What are the advantages of Clean in Place?
Nowadays, CIP systems are an indispensable part of modern manufacturing processes, not only in the beverage but also in food industries.
The main advantage of the CIP technology is the omission of disassembly of your installation to clean and disinfect product-leading parts. It is therefore also suitable for cleaning difficult-to-access parts of your system where manual cleaning methods are no longer effective or feasible. With the aid of built-in nozzles or spray balls, even large-volume system parts such as tanks can be cleaned without complete flooding.
The advantages of the CIP process
The CIP process is a fully automated process and should be perfectly adapted to your production facility. As a result, a consistently high, reproducible cleaning quality is achieved with minimal use of time and resources – this drastically reduces cleaning costs. Depending on the cleaning tasks and the circulation volume of the cleaning circuit, it may make sense to reuse the cleaning media – referred to as backstacking. As it is individually tailored to your requirements, you can optimize the consumption of cleaning media and wastewater volumes, making a meaningful contribution to protecting the environment.
The ever-increasing energy costs make CIP systems even more interesting for you. With its energy-efficient mode of operation, the process simultaneously reduces the energy consumption of both the process and the cleaning system. Hence you can achieve very good cleaning results and optimized energy consumption for your plant simply by relying on the automated processes and precisely adjustable parameters such as temperature, flow rate and duration of action of the cleaning medium.
Advantages at a glance
- Increased product safety due to reproducible cleaning results
- Increased employee safety compared to manual cleaning processes
- Savings in valuable resources such as water, time, energy and chemicals
- Reduction of cleaning costs (lower wastewater costs, reduced process costs, shorter cycle times)
Your challenge
As a manufacturer it is key to prevent contamination and harmful microorganisms that can result from remaining product residues. Increasingly stringent laws and hygiene regulations require detailed documentation of cleanliness of your equipment and cleaning processes. Naturally, you only want to interrupt your production process as short as absolutely necessary for cleaning and must avoid downtimes as far as possible in order to operate economically.
Process hygiene is therefore a major issue, especially in the beverage and food industry. Industry experts have long been concerned with topics such as reproducible cleaning, resource savings, and food safety.
LOEHRKE's automatic CIP cleaning can bypass cumbersome and expensive manual cleaning and improve the hygiene of your systems. Our CIP products are also suitable for open systems (e.g. storage tanks) to increase efficiency. Successful cleaning improves the microbiological safety of your products and is an important component of your quality assurance.
Of course, we will examine each application individually and determine the cleaning options with your field of use in mind. Usually, mainly piping systems, tanks, vessels, heat exchangers, reactors and special tanks are cleaned with in-place cleaning processes.
We will be happy to advise you on your individual needs and help you optimize your parameters.
We are not in a direct partnership with chemical distributors, allowing us to deliver cleaning technology that can be used with a variety of chemicals. The choice is always yours.
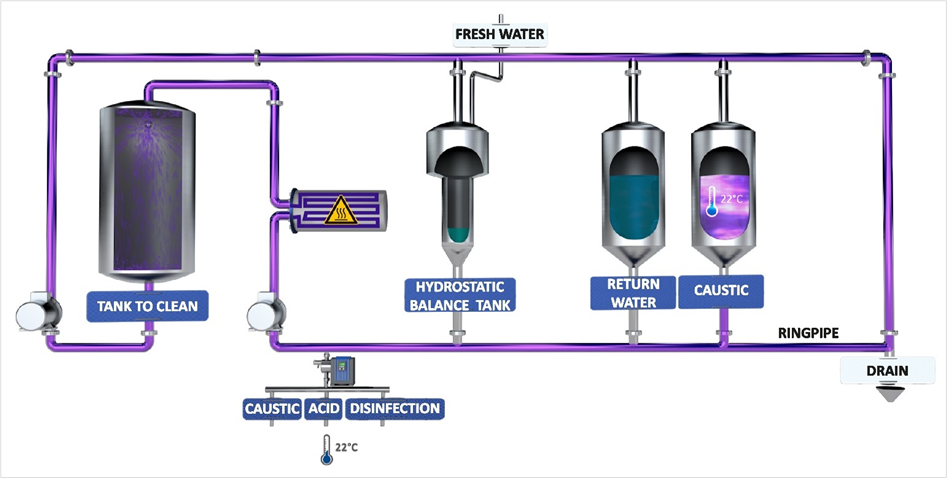
The CIP process in detail
Traditionally, a 5-step process is often used for CIP cleaning:
- Pre-rinse (mostly with return water).
- Caustic (hot)
- Intermediate rinse with fresh water
- Acid (hot or cold)
- Final rinse with fresh water
According to this concept, pre-rinsing is usually followed by cleaning with caustic solution, which is then rinsed out again using fresh water, and cleaning is completed with acid. Acid residues are removed with a final rinsing step (final rinse) using fresh water. All cleaning steps are carried out in all pipelines of the cleaning object.
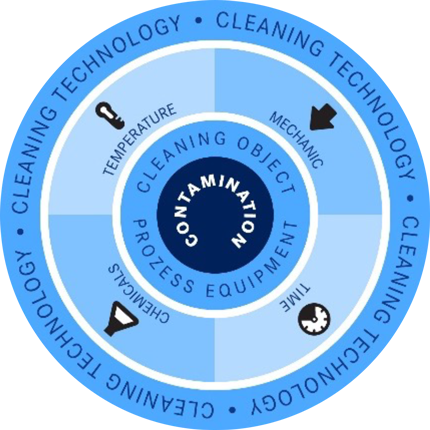
Depending on the influencing factors of temperature, time (cleaning duration), use of chemicals and mechanical action (flow), there are different variations in the cleaning process. The interrelation of these factors is described in the so-called Sinner's circle.
If one of the factors is changed, it inevitably results in an adjustment of the other parameters. For example, an increase in the cleaning temperature reduces the required cleaning agent concentration or the time required for cleaning, or vice versa.
LOEHRKE CIP cleaning systems are optimally adapted to your individual requirements and pursue a simple goal: maximum cleaning effect with minimal use of resources.
While LOEHRKE CIP already offers numerous optimizations compared to classic CIP methods further efficiency increase can be achieved. Through the addition and clever combination of several modules that are tailored to your individual local needs we can improve effectiveness and efficiency.
Together we will work out which of our CIP processes is the right one for you. Based on the local conditions and your requirements, LOEHRKE will provide you with a CIP system that is specifically tailored to your needs. In addition to offering you a new CIP Solution, we are also happy to review an existing CIP process with you and explain possible optimization measures.
Differentiation between Cleaning in Place (CIP) and Sterilization in Place (SIP)
Sterilization in Place (SIP) is a steam or hot water sterilization at 120 to 130 degrees Celsius, which is only possible under pressure. During sterilization, all microorganisms, including spores, viruses and enzymes, are killed by heat.
SIP replaces disinfection after a completed cleaning and is used, for example, for UHT systems in the dairy industry.
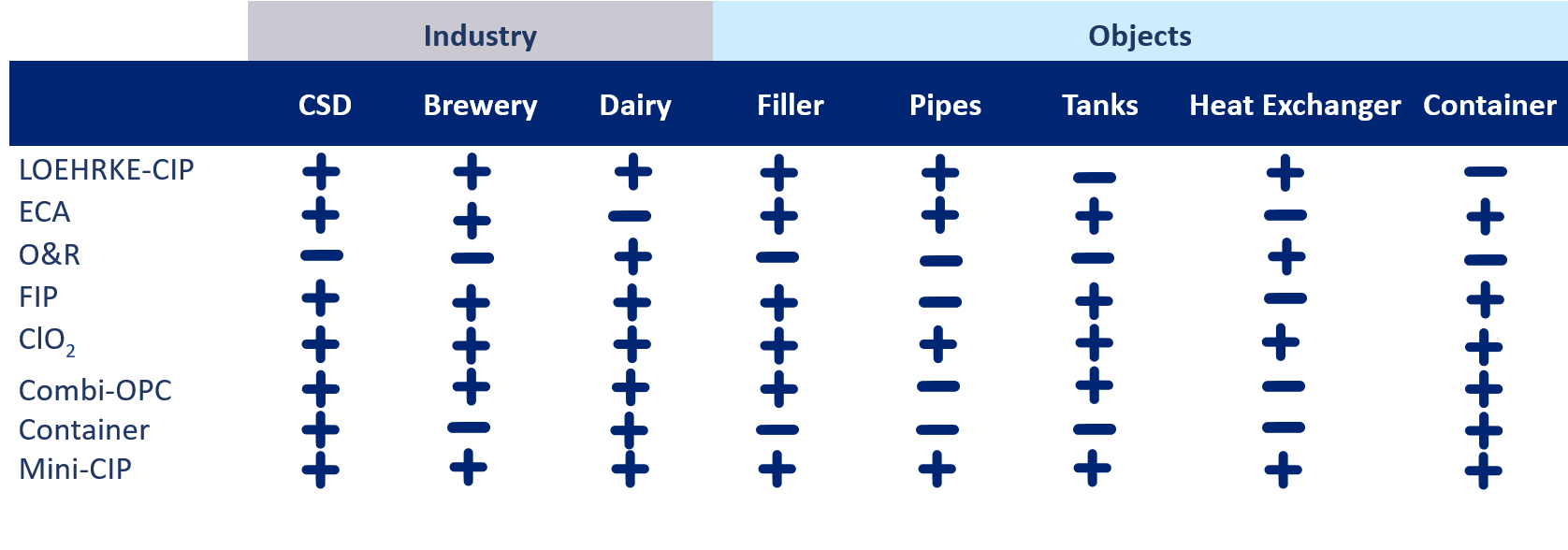
Our LOEHRKE CIP plants and modules
Whether you need a module or a new system, we will find the right solution for you. For further information, please click on the individual sections of our overview.
*Plus/ Minus do not indicate general functionality. The table shows the individual applications with the advantages for the respective option.
FAQ
CIP systems are used in the food, beverage, cosmetics and pharmaceutical industries. After production, product residues and microorganisms remain in the production equipment. Lime and grease deposits also lead to contamination, which can be removed by a Clean in Place system. Cleaning results in an increase in product safety and product quality. This can enable a longer minimum shelf life of the products due to improved microbiological safety.
After assessing requirements in your production and receiving all relevant customer data such as drawings, layouts, and specifications, we define the appropriate areas for CIP cleaning, taking into account your personal needs for the plant. This is also where we decide which CIP processes are most suitable for your production plant. For example, systems for circulation cleaning can principally be set up centrally or decentralized. The right choice depends on various influencing factors. Both criteria as well as the decision whether a new system is required or whether a retro-fit would be sufficient influence the cost. We will be happy to prepare an individual calculation for you.
Product residues in food and beverage production are a source of microbial contamination in the industry. Lime and grease deposits also cause contamination of production equipment, which can be cleaned by a Clean In Place system. The key advantage of CIP cleaning is that the process takes place fully automatically and without disassembling the system. This allows high reproducibility and automatic logging of cleaning procedures. Product residues and microorganisms are reliably removed from the production line, resulting in higher product safety and product quality.
Depending on your local conditions and requirements the selection of which CIP- process is right for your needs may vary.
If transport distances are short and cleaning tasks are very similar or the same, the central CIP process is suitable, for which only one system is required. Large companies that require individual cleaning processes should use the decentralized CIP process. Multiple and separate systems eliminate cross-contamination and allow flexible program control.
The "lost" cleaning is particularly suitable for sensitive (keyword allergen contamination), as well as heavily contaminated areas or if cleaning is done only rarely. In this case, the cleaning medium used is discarded at the end of the cleaning process. Stack cleaning, in which the cleaning medium is "stacked" at the end, i. e. stored in a tank until the next use, is suitable for frequent cleaning of similar product residues, for example in a dairy. Of course, a certain investment is necessary to make the stacking possible. We are happy to carry out a non-binding profitability calculation for you.
Our mobile CIP system, the LOEHRKE MiniCIP, ensures reliable cleaning wherever a large CIP system is not economical. MiniCIP enables you to professionally CIP clean in small sections and also perform individual cleaning in critical process sections.
With its 300 l storage tank, automatic dosing of up to 3 cleaning/disinfection chemicals and a feed pump of up to 10 m³/h, it is typically used for cleaning and disinfection of small filling systems, pipes up to DN 40, special tanks, vessels, reactors, and much more.
If you choose the option of "retrofitting your old system", we will be sure to carefully check which components can be reused if necessary and which should be replaced.
Our specialists will be happy to advise you on whether a completely new system or a retrofit is the better option for you. Together we will find the optimal solution to your individual application.
The individual components of a CIP system may seem like standard products at first. However, it all comes down to the design of the system, its integration into your plant, and the overall process of understanding and equipping the system according to the specific requirements for your production facility. This therefore makes each system a completely customized solution. Once the system is to be integrated into your unique facility, it becomes a custom project that requires experienced installation and configuration by specialists.
The residue to be cleaned is relevant for the type of cleaning agent, the temperature and the mechanics required. Organic contaminants (proteins, fats) are typically cleaned with lye. Inorganic residues (e. g. lime deposits due to high water hardness or milk stone) are typically cleaned with acid. Often a combination of both is the right solution. Concentrations, temperatures and flow rates of the cleaning solutions must be carefully selected to optimize performance.
For a fully functional CIP system, the following media are generally required in sufficient quantities in each case: Potable water, detergent and steam, heating water or electricity (to reach the required temperature). In addition, a sufficient waste water system must be available.